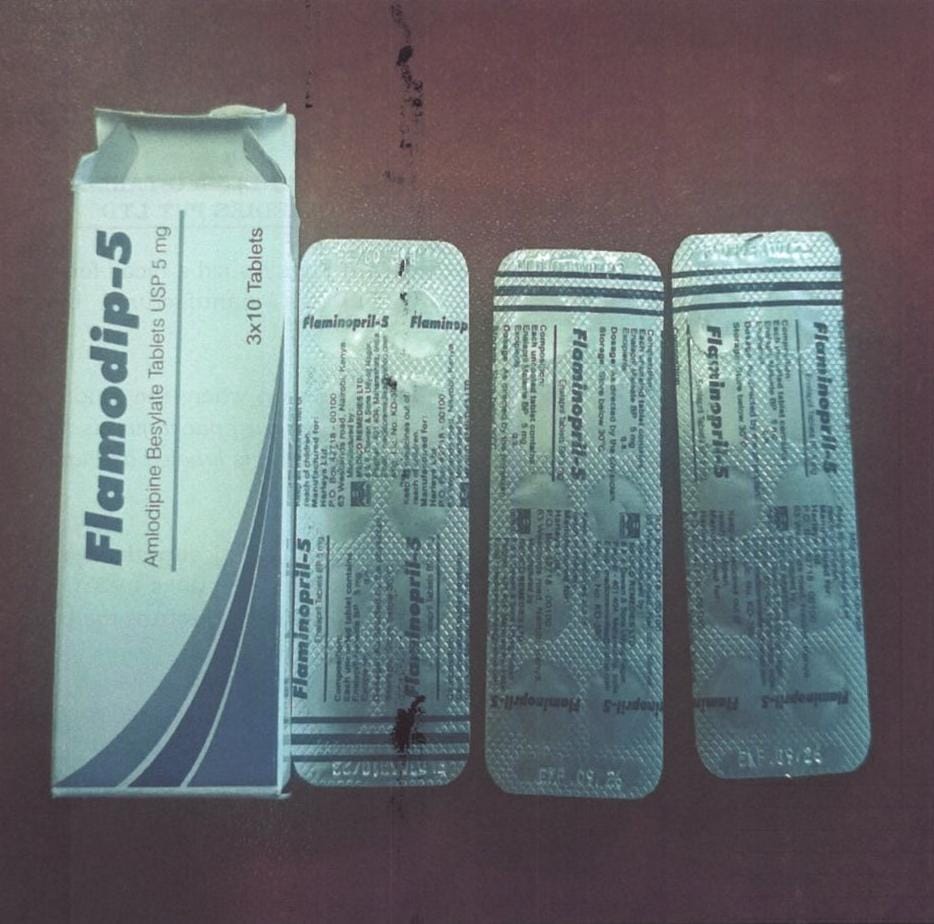
The Pharmacy and Poisons Board has issued a recall for Flamodip (Amlodipine) 5mg Tablets, Batch No. FLD303, manufactured by Medico Remedies Pvt Ltd.
In a statement the Board has further advised all pharmaceutical outlets , healthcare professionals and members of public to stop distribution, sale, issuing or use of the product batch and return the product to their nearest healthcare facility or respective supplier.
The Board notes that the recall has been initiated due to a labelling error, where the product’s label does not accurately reflect its contents.
“The secondary packaging is labelled as Flamodip – 5 (Amlodipine), while the primary packaging is labelled as Flamonopri-5 (Enalapril)” The Board stated.
Additionally, the Board has encouraged the public to promptly report any suspected cases of sub-standard medicine or adverse drug reactions to the nearest healthcare facility or Pharmacy and Poisons Board through https://pv.pharmacyboardkenya.org/users/mpublic.
The public can also report through USSD code *271# or call 0795743049 or email the board via pv@ppb.go.ke or pms@ppb.go.ke.
Flamodip is a prescription medication used to treat conditions such as high blood pressure, angina, or ischemic heart disease.
It belongs to the group of cardiovascular drugs, used mainly in cases of hypertension or myocardial ischemia accompanied by stable angina pectoris.
During the course of treatment with Flamedip, patients need to follow the instructions of the specialist to get rid of the disease soon.